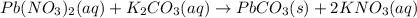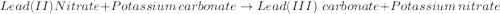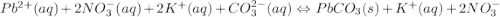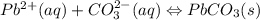Use the equation editor or "insert chemistry - wiris editor" to write the balanced molecular chemical equation for the reaction of aqueous 0.13 m lead (ii) nitrate, with 0.19 m potassium carbonate. you may need to consult appendix e to determine the states of each reactant and product. assume any insoluble products are completely insoluble.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

You know the right answer?
Use the equation editor or "insert chemistry - wiris editor" to write the balanced molecular chemica...
Questions







Mathematics, 07.09.2020 02:01

Mathematics, 07.09.2020 02:01


Mathematics, 07.09.2020 02:01


English, 07.09.2020 02:01