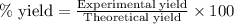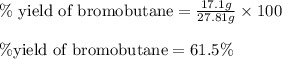Chemistry, 30.11.2019 01:31 akatsionis25
Use the following reaction: c4h9oh + nabr + h2so4 c4h9br + nahso4 + h2o if 15.0 g of c4h9oh react with 22.4 g of nabr and 32.7 g of h2so4 to yield 17.1 g of c4h9br, what is the percent yield of this reaction? remember: percent yield is your (experimental yield/theoretical yield)x100.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the root word engage means “to connect with something,” what does the word disengage mean in the following sentence? he disengaged the gears by stepping on the clutch pedal.a.added more engine powerb.activated a connection to the pedalc.stalled the engined.released a connection to the pedal
Answers: 1

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1
You know the right answer?
Use the following reaction: c4h9oh + nabr + h2so4 c4h9br + nahso4 + h2o if 15.0 g of c4h9oh react w...
Questions

Mathematics, 19.05.2021 21:30

History, 19.05.2021 21:30








Mathematics, 19.05.2021 21:30


History, 19.05.2021 21:30

Mathematics, 19.05.2021 21:30

Biology, 19.05.2021 21:30


English, 19.05.2021 21:30


Chemistry, 19.05.2021 21:30

Mathematics, 19.05.2021 21:30

History, 19.05.2021 21:30

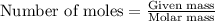 .....(1)
.....(1)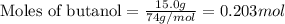
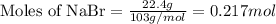
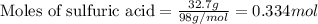
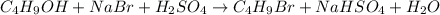
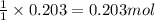 of bromobutane
of bromobutane