
Chemistry, 28.11.2019 04:31 dbn4everloved8
A1.00 g sample of n-hexane (c6h14) undergoes complete combustion with excess o2 in a bomb calorimeter. the temperature of the 1502 g of water surrounding the bomb rises from 22.64°c to 29.30°c. the heat capacity of the hardware component of the calorimeter (everything that is not water) is 4042 j/°c. what is δu for the combustion of n-c6h14? one mole of n-c6h14 is 86.1 g. the specific heat of water is 4.184 j/g·°c.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2


Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1
You know the right answer?
A1.00 g sample of n-hexane (c6h14) undergoes complete combustion with excess o2 in a bomb calorimete...
Questions







Mathematics, 02.09.2021 07:10

Mathematics, 02.09.2021 07:10

Mathematics, 02.09.2021 07:10

Biology, 02.09.2021 07:10



Spanish, 02.09.2021 07:10

Health, 02.09.2021 07:10

Biology, 02.09.2021 07:10

English, 02.09.2021 07:10




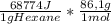 = -5,921x10⁶J/mol
= -5,921x10⁶J/mol

