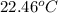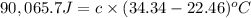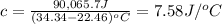Chemistry, 27.11.2019 06:31 lineaeriksen
A2.15g sample of benzene (c_6h_6) is burned in a bomb calorimeter, and the temperature rises from 22.46 degree c to 34.34 degree c. calculate the heat capacity of the bomb calorimeter. note the following thermochemical equation: c_6h_6(i) + 15/2 o_2 (g) rightarrow 6co_2 (g) + 3h_2o (g) delta h degree = -3267.5 kj

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
You know the right answer?
A2.15g sample of benzene (c_6h_6) is burned in a bomb calorimeter, and the temperature rises from 22...
Questions

Mathematics, 23.12.2020 03:50



Business, 23.12.2020 03:50

Mathematics, 23.12.2020 03:50



Mathematics, 23.12.2020 03:50








Mathematics, 23.12.2020 03:50


Biology, 23.12.2020 03:50

French, 23.12.2020 03:50

Mathematics, 23.12.2020 03:50

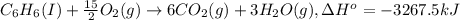
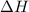 = enthalpy change = -3267.5 kJ/mol
= enthalpy change = -3267.5 kJ/mol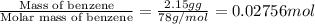
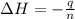
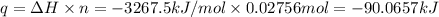
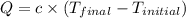
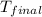 = final temperature =
= final temperature = 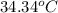
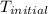 = initial temperature =
= initial temperature =