
Chemistry, 27.11.2019 01:31 lattimorekeonna1
If 60.0 grams of carbonic acid are sealed in a 2.00 l soda bottle at room temperature (298 k) and decompose completely via the equation below, what would be the final pressure of carbon dioxide assuming it had the full 2.00 l in which to expand? h₂co₃(aq) → h₂o(l) + co₂(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2


Chemistry, 23.06.2019 06:00
What volume of 0.500 mol/l hydrochloric acid, hci (aq) is required to react completely with 1.00 g of aluminum hydroxide, ai(oh)3 (s)?
Answers: 1

Chemistry, 23.06.2019 07:00
In order for a high temperature boiler or steam engine to produce superheated water, or steam: the heat source must be greater than 100°c the water must be permitted to evaporate quickly the system must be sealed and become pressurized above atmospheric pressure the vapor pressure must be kept below 760 mm(hg)
Answers: 1
You know the right answer?
If 60.0 grams of carbonic acid are sealed in a 2.00 l soda bottle at room temperature (298 k) and de...
Questions





Mathematics, 04.02.2020 14:03





World Languages, 04.02.2020 14:03



Geography, 04.02.2020 14:03





English, 04.02.2020 14:04


Biology, 04.02.2020 14:04

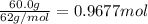
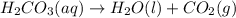
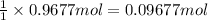 of carbon dioxide
of carbon dioxide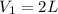
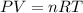 (ideal gas law)
(ideal gas law)


