
Chemistry, 26.11.2019 21:31 rebeckas0102
For the following reactions, the δh° rxn is not equal to δh° f for the product except for
a. h2o (l) + 1/2 o2 (g) → h2o2(l)
b. n2 (g) + o2 (g) → 2no (g)
c. 2h2 (g) + o2 (g) → 2h2o (g)
d. 2h2 (g) + o2 (g) → 2h2o (l)
e. 2c(s, graphite) + 2h2(g) → c2h4 (g)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1
You know the right answer?
For the following reactions, the δh° rxn is not equal to δh° f for the product except for
Questions










English, 03.10.2019 00:00

Mathematics, 03.10.2019 00:00

History, 03.10.2019 00:00








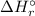 ) is the change in enthalpy associated with a given chemical reaction. It can be calculated by the standard enthalpies of formation of the products and reactants.
) is the change in enthalpy associated with a given chemical reaction. It can be calculated by the standard enthalpies of formation of the products and reactants. 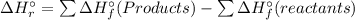
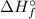 ) of an elements that is present in its standard state is zero.
) of an elements that is present in its standard state is zero.![\Delta H_{r}^{\circ} = \left [\Delta H_{f}^{\circ} (H_{2}O_{2}, l) \right ] - \left [\Delta H_{f}^{\circ} (H_{2}O, l)+ 1/2\times \Delta H_{f}^{\circ} (O_{2}, g)\right]](/tpl/images/0392/0323/5735d.png)
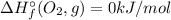
![\Delta H_{r}^{\circ} = \left [\Delta H_{f}^{\circ} (H_{2}O_{2}, l) \right ] - \left [\Delta H_{f}^{\circ} (H_{2}O, l)\right] \neq \Delta H_{f}^{\circ} (Products)](/tpl/images/0392/0323/60595.png)
![\Delta H_{r}^{\circ} = \left [2\times \Delta H_{f}^{\circ} (NO, g) \right ] - \left [\Delta H_{f}^{\circ} (N_{2}, g)+\Delta H_{f}^{\circ} (O_{2}, g)\right]](/tpl/images/0392/0323/c62af.png)
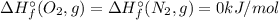
![\Delta H_{r}^{\circ} = \left [2\times \Delta H_{f}^{\circ} (NO, g) \right ] \neq \Delta H_{f}^{\circ} (Product, NO)](/tpl/images/0392/0323/39174.png)
![\Delta H_{r}^{\circ} = \left [2\times \Delta H_{f}^{\circ} (H_{2}O, g) \right ] - \left [2\times \Delta H_{f}^{\circ} (H_{2}, g)+\Delta H_{f}^{\circ} (O_{2}, g)\right]](/tpl/images/0392/0323/6e78e.png)
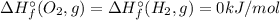
![\Delta H_{r}^{\circ} = \left [2\times \Delta H_{f}^{\circ} (H_{2}O, g) \right ] \neq \Delta H_{f}^{\circ} (Product, H_{2}O)](/tpl/images/0392/0323/b5284.png)
![\Delta H_{r}^{\circ} = \left [2\times \Delta H_{f}^{\circ} (H_{2}O, l) \right ] - \left [2\times \Delta H_{f}^{\circ} (H_{2}, g)+\Delta H_{f}^{\circ} (O_{2}, g)\right]](/tpl/images/0392/0323/a373f.png)
![\Delta H_{r}^{\circ} = \left [2\times \Delta H_{f}^{\circ} (H_{2}O, l) \right ] \neq \Delta H_{f}^{\circ} (Product, H_{2}O)](/tpl/images/0392/0323/02a0f.png)
![\Delta H_{r}^{\circ} = \left [\Delta H_{f}^{\circ} (C_{2}H_{4}, g) \right ] - \left [2\times \Delta H_{f}^{\circ} (C, s graphite)+2\times \Delta H_{f}^{\circ} (H_{2}, g)\right]](/tpl/images/0392/0323/d87f3.png)
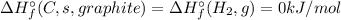
![\Delta H_{r}^{\circ} = \left [\Delta H_{f}^{\circ} (C_{2}H_{4}, g) \right ] = \Delta H_{f}^{\circ} (Product, C_{2}H_{4})](/tpl/images/0392/0323/5f4af.png)



