Chemistry, 26.11.2019 21:31 Ciarrathereal
Achemist measures the energy change δh during the following reaction: c3h8 (g) +5o2 (g) →3co2 (g) +4h2o (l) =δh−2220.kj use the information to answer the following questions.
this reaction
endothermic.
exothermic.
suppose
81.0g
of
c3h8
react.
will any heat be released or absorbed?
yes, absorbed.
yes, released.
no.
if you said heat will be released or absorbed in the second part of this question, calculate how much heat will be released or absorbed.
kj
round your answer to
3
significant digits.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1
You know the right answer?
Achemist measures the energy change δh during the following reaction: c3h8 (g) +5o2 (g) →3co2 (g) +...
Questions



Mathematics, 06.04.2020 18:42

English, 06.04.2020 18:42



Mathematics, 06.04.2020 18:42

Mathematics, 06.04.2020 18:42


History, 06.04.2020 18:42

Chemistry, 06.04.2020 18:42






Mathematics, 06.04.2020 18:42

Mathematics, 06.04.2020 18:42

History, 06.04.2020 18:42

English, 06.04.2020 18:42

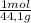 ×
×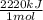 = 4,08x10³ kJ
= 4,08x10³ kJ