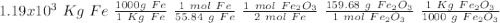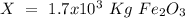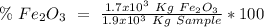Chemistry, 23.11.2019 00:31 mjweed3381
One of the reactions that occurs in a blast furnace, in which iron ore is converted to cast iron, is fe2o3 + 3co → 2fe + 3co2 suppose that 1.19 × 103 kg of fe is obtained from a 1.90 × 103 kg sample of fe2o3. assuming that the reaction goes to completion, what is the percent purity of fe2o3 in the original sample?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

You know the right answer?
One of the reactions that occurs in a blast furnace, in which iron ore is converted to cast iron, is...
Questions


Mathematics, 27.03.2020 21:45

English, 27.03.2020 21:45

Mathematics, 27.03.2020 21:45




History, 27.03.2020 21:45

Mathematics, 27.03.2020 21:45

Mathematics, 27.03.2020 21:45

Mathematics, 27.03.2020 21:45


Mathematics, 27.03.2020 21:45






Social Studies, 27.03.2020 21:46

Physics, 27.03.2020 21:46

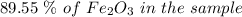
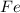 to grams of
to grams of 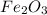 .
.