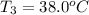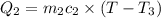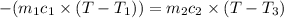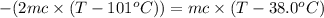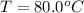Chemistry, 23.11.2019 00:31 sidallen05
One piece of copper jewelry at 101°c has twice the mass of another piece, which is at 38.0°c. both pieces are placed inside a calorimeter of negligible heat capacity. what is the final temperature inside the calorimeter (c of copper=0.387 j/g. k)?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
You know the right answer?
One piece of copper jewelry at 101°c has twice the mass of another piece, which is at 38.0°c. both p...
Questions

Computers and Technology, 28.08.2020 05:01

Mathematics, 28.08.2020 05:01

Mathematics, 28.08.2020 05:01




Business, 28.08.2020 05:01






Mathematics, 28.08.2020 05:01

History, 28.08.2020 05:01




Biology, 28.08.2020 05:01


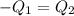
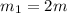
 = 0.387 J/g.K
= 0.387 J/g.K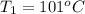
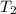 =T
=T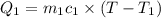
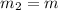
 = 0.387 J/g.K
= 0.387 J/g.K