Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:00
The study of witch tree monkeys feed in is part of the science life
Answers: 1


Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1
You know the right answer?
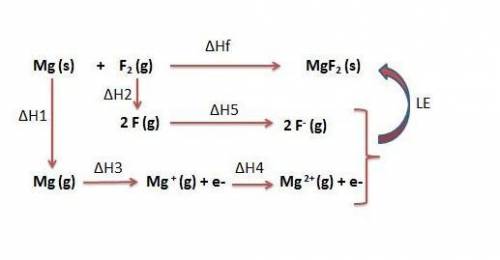
Use the following to calculate h°lattice of mgf2. mg(s) mg(g) h° = 148 kj f2(g) 2 f(g) h° = 159 kj m...
Questions

Mathematics, 21.01.2021 20:40



History, 21.01.2021 20:40


History, 21.01.2021 20:40

Computers and Technology, 21.01.2021 20:40

Mathematics, 21.01.2021 20:40


Mathematics, 21.01.2021 20:40

Mathematics, 21.01.2021 20:40

History, 21.01.2021 20:40


Mathematics, 21.01.2021 20:40


Mathematics, 21.01.2021 20:40

Mathematics, 21.01.2021 20:40


History, 21.01.2021 20:40

Chemistry, 21.01.2021 20:40