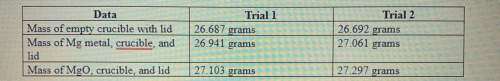
1. calculate the actual yield of magnesium oxide for each trial.
trial 1:
trial...

Chemistry, 22.11.2019 04:31 cloeybrown
1. calculate the actual yield of magnesium oxide for each trial.
trial 1:
trial 2:
2. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial.
trial 1:
trial 2:
3. determine the percent yield of mgo for your experiment for each trial.
trial 1:
trial 2:
4. determine the average percent yield of mgo for the two trials.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1


Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1
You know the right answer?
Questions


Mathematics, 24.06.2019 19:30

Mathematics, 24.06.2019 19:30

Mathematics, 24.06.2019 19:30

Mathematics, 24.06.2019 19:30


Mathematics, 24.06.2019 19:30

Arts, 24.06.2019 19:30

Mathematics, 24.06.2019 19:30



History, 24.06.2019 19:30

Mathematics, 24.06.2019 19:30


Physics, 24.06.2019 19:30




English, 24.06.2019 19:30



