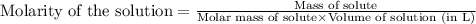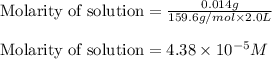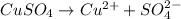Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 01:30
List and describe the neurological effects of the vocs and other air pollutants,as described by dr.theo colborn
Answers: 2
You know the right answer?
The concentration of copper(ii) sulfate in one brand of soluble plant fertilizer is 0.07% by mass. i...
Questions

History, 27.07.2019 18:30

History, 27.07.2019 18:30

Mathematics, 27.07.2019 18:30







Mathematics, 27.07.2019 18:30


Chemistry, 27.07.2019 18:30


History, 27.07.2019 18:30






Social Studies, 27.07.2019 18:30

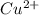 ions in the given amount of sample is
ions in the given amount of sample is 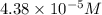
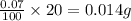 of copper (II) sulfate is present.
of copper (II) sulfate is present.