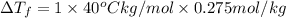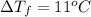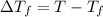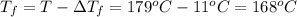Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2
You know the right answer?
Assume the molality of isoborneol in your product is 0.275 mol/kg. what is the melting point of your...
Questions



Mathematics, 15.04.2020 17:18








Social Studies, 15.04.2020 17:18


Computers and Technology, 15.04.2020 17:18

Biology, 15.04.2020 17:18






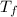 = ?
= ?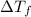
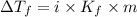
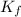 = The freezing point depression constant
= The freezing point depression constant