
Chemistry, 21.11.2019 03:31 briannamaee13
Areaction was performed in which 0.55 g of 2‑naphthol was reacted with a slight excess of allyl bromide to make 0.59 g of allyl 2‑naphthyl ether. calculate the theoretical yield and percent yield for this reaction. allyl bromide has a density of 1.40 g/ml .

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:10
Nuclear fusion is the source of energy for stars. besides hydrogen, which other element is most likely also common in stars?
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

You know the right answer?
Areaction was performed in which 0.55 g of 2‑naphthol was reacted with a slight excess of allyl brom...
Questions


Mathematics, 19.03.2021 02:00

Arts, 19.03.2021 02:00

Computers and Technology, 19.03.2021 02:00

Mathematics, 19.03.2021 02:00





Mathematics, 19.03.2021 02:00


History, 19.03.2021 02:00

Mathematics, 19.03.2021 02:00


Mathematics, 19.03.2021 02:00



Mathematics, 19.03.2021 02:00


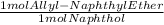 * 184 gEther/mol = 0.7028 g
* 184 gEther/mol = 0.7028 g

