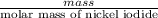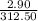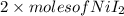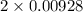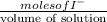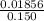Chemistry, 21.11.2019 03:31 maria051002camp
Uppose of nickel(ii) iodide is dissolved in of a aqueous solution of potassium carbonate. calculate the final molarity of iodide anion in the solution. you can assume the volume of the solution doesn't change when the nickel(ii) iodide is dissolved in it. be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3
You know the right answer?
Uppose of nickel(ii) iodide is dissolved in of a aqueous solution of potassium carbonate. calculate...
Questions

Mathematics, 22.07.2020 19:01





Chemistry, 22.07.2020 19:01


Biology, 22.07.2020 19:01



Mathematics, 22.07.2020 19:01



English, 22.07.2020 19:01

Mathematics, 22.07.2020 19:01


Computers and Technology, 22.07.2020 19:01



World Languages, 22.07.2020 19:01

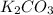 is added into the solution then it will not affect the
is added into the solution then it will not affect the 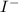 concentration.
concentration.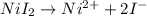
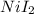 =
=