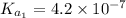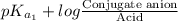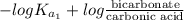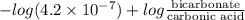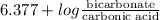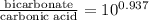The ph of blood is 7.35. it is maintained in part by the buffer system composed of carbonic acid (h2co3) and the bicarbonate (hydrogen carbonate, hco3-) ion. what is the ratio of [bicarbonate]/[carbonic acid] at this ph? for carbonic acid, ka1 = 4.2 10-7.a) [bicarbonate]/[carbonic acid] = 0.11d) [bicarbonate]/[carbonic acid] = 9.4b) [bicarbonate]/[carbonic acid] = 0.38e) none of the above ratios is correct. c) [bicarbonate]/[carbonic acid] = 2.65

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
The ph of blood is 7.35. it is maintained in part by the buffer system composed of carbonic acid (h2...
Questions

Mathematics, 04.02.2021 16:00



Mathematics, 04.02.2021 16:00

English, 04.02.2021 16:00



World Languages, 04.02.2021 16:00

Computers and Technology, 04.02.2021 16:00

Mathematics, 04.02.2021 16:00

Chemistry, 04.02.2021 16:00

Mathematics, 04.02.2021 16:00

Social Studies, 04.02.2021 16:00

Mathematics, 04.02.2021 16:00


Engineering, 04.02.2021 16:00


Mathematics, 04.02.2021 16:00

Mathematics, 04.02.2021 16:00

Mathematics, 04.02.2021 16:00