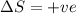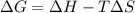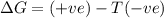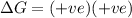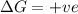Chemistry, 20.11.2019 23:31 annjetero2oy23ay
Consider the endothermic reaction: n2 (g) 2 h2 (g) → n2h4 (l) the entropy change of this reaction is and the enthalpy change is so at a very high temperature, this reaction is probably consider the endothermic reaction: n2 (g) 2 h2 (g) → n2h4 (l) the entropy change of this reaction is and the enthalpy change is so at a very high temperature, this reaction is probably unfavorable; unfavorable; nonspontaneous unfavorable; favorable; spontaneous favorable; unfavorable; spontaneous favorable; unfavorable; nonspontaneous unfavorable; unfavorable; spontaneous

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 23.06.2019 01:00
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2

Chemistry, 23.06.2019 04:31
Chemical engineering who specializes in negotiating for large purchases and instructing customers in use of the products are
Answers: 1
You know the right answer?
Consider the endothermic reaction: n2 (g) 2 h2 (g) → n2h4 (l) the entropy change of this reaction i...
Questions


Health, 25.06.2019 02:30


English, 25.06.2019 02:30


Mathematics, 25.06.2019 02:30

English, 25.06.2019 02:30

History, 25.06.2019 02:30

Social Studies, 25.06.2019 02:30


History, 25.06.2019 02:30


Biology, 25.06.2019 02:30



Computers and Technology, 25.06.2019 02:30

Mathematics, 25.06.2019 02:30


Mathematics, 25.06.2019 02:30

Advanced Placement (AP), 25.06.2019 02:30

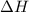 for Endothermic reaction is positive and
for Endothermic reaction is positive and 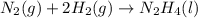
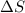 is negative as the randomness decreases when gases convert into liquid.
is negative as the randomness decreases when gases convert into liquid. 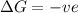 and favourable conditions are
and favourable conditions are 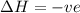 and
and