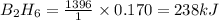Chemistry, 19.11.2019 22:31 ronaldotheexplorer12
According to the following reaction, how much energy is evolved during the reaction of 32.5 g b2h6 and 72.5 g cl2? the molar mass of b2h6 is 27.67 g/mol. b2h6(g) + 6 cl2(g) → 2 bcl3(g) + 6 hcl(g) δh°rxn = -1396 kj according to the following reaction, how much energy is evolved during the reaction of 32.5 g b2h6 and 72.5 g cl2? the molar mass of b2h6 is 27.67 g/mol. b2h6(g) + 6 cl2(g) → 2 bcl3(g) + 6 hcl(g) δh°rxn = -1396 kj a) 1640 kj b) 1430 kj c) 429 kj d) 3070 kj e) 238 kj.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1
You know the right answer?
According to the following reaction, how much energy is evolved during the reaction of 32.5 g b2h6 a...
Questions

Mathematics, 09.12.2020 20:00


Mathematics, 09.12.2020 20:00

Mathematics, 09.12.2020 20:00

Mathematics, 09.12.2020 20:00


Mathematics, 09.12.2020 20:00

Chemistry, 09.12.2020 20:00




Mathematics, 09.12.2020 20:00

Mathematics, 09.12.2020 20:00


Mathematics, 09.12.2020 20:00

History, 09.12.2020 20:00


Mathematics, 09.12.2020 20:00

Physics, 09.12.2020 20:00

History, 09.12.2020 20:00

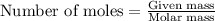 .....(1)
.....(1)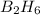 :
: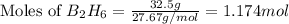
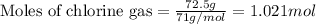
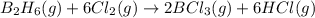
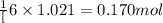 of
of