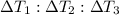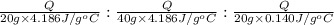Water’s specific heat capacity is 4.186 joules/gram degree celsius. mercury’s specific heat capacity is 0.140 joules/gram degree celsius.
water and mercury are put into three identical bowls:
bowl a contains 20 grams of water.
bowl b contains 40 grams of water.
bowl c contains 20 grams of mercury.
the bowls start at the same temperature, and then the same amount of heat is added to each bowl. order the bowls from coolest to warmest, based on their final temperatures.
bowl a

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an oxidation-reduction reaction, oxidation is what happens when a reactant
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1

Chemistry, 23.06.2019 04:31
One student said that the investigation was not valid (a fair test). write a plan for the investigation that includes improvements to the method and apparatus
Answers: 1
You know the right answer?
Water’s specific heat capacity is 4.186 joules/gram degree celsius. mercury’s specific heat capacity...
Questions








Mathematics, 20.10.2019 21:20


English, 20.10.2019 21:20

History, 20.10.2019 21:20

Biology, 20.10.2019 21:20

Mathematics, 20.10.2019 21:20

History, 20.10.2019 21:20


Mathematics, 20.10.2019 21:20


Chemistry, 20.10.2019 21:20

Chemistry, 20.10.2019 21:20

Physics, 20.10.2019 21:20

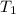
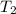
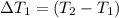
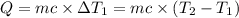
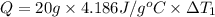
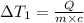
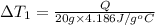 ..[1]
..[1]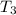
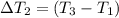
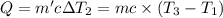
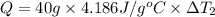
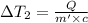
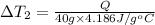 ..[2]
..[2]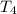
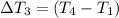
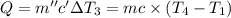
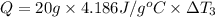
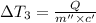
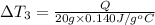 ..[3]
..[3]