
Chemistry, 18.11.2019 20:31 noahdeem135
Industrial production of nitric acid, which is used in many products including fertilizers and explosives, approaches 10 billion kg per year worldwide. the first step in its production is the exothermic oxidation of ammonia, represented by the following equation. 4 nh3(g) + 5 o2(g) → 4 no(g) + 6 h2o(g) δh⁰rxn = −902.0 kj if this reaction is carried out using 7.056 ✕ 103 g nh3 as the limiting reactant, what is the change in enthalpy?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2


You know the right answer?
Industrial production of nitric acid, which is used in many products including fertilizers and explo...
Questions

Mathematics, 03.02.2020 21:50


Mathematics, 03.02.2020 21:50


Mathematics, 03.02.2020 21:50

Mathematics, 03.02.2020 21:50


History, 03.02.2020 21:50




History, 03.02.2020 21:50

Mathematics, 03.02.2020 21:50

Social Studies, 03.02.2020 21:50

English, 03.02.2020 21:50

Mathematics, 03.02.2020 21:50

Advanced Placement (AP), 03.02.2020 21:50



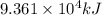
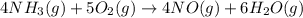
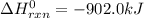
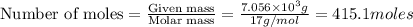
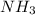 produces = 902.0 kJ of energy
produces = 902.0 kJ of energy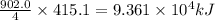 of energy
of energy

