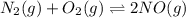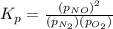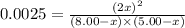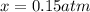Chemistry, 18.11.2019 20:31 sierravick123owr441
Nitric oxide is formed in automobile exhaust when nitrogen and oxygen in air react at high temperatures. n2(g) + o2(g) 2no(g)the equilibrium constant kp for the reaction is 0.0025 at 2127�c. if a container is charged with 8.00 atm of nitrogen and 5.00 atm of oxygen and the mixture is allowed to reach equilibrium, what will be the equilibrium partial pressure of nitrogen? a) 0.16 atm b) 0.31 atm c) 3.1 atm d) 7.7 atm e) 7.8 atm

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
You know the right answer?
Nitric oxide is formed in automobile exhaust when nitrogen and oxygen in air react at high temperatu...
Questions

History, 23.09.2021 23:40

Mathematics, 23.09.2021 23:40


Biology, 23.09.2021 23:40

Chemistry, 23.09.2021 23:40

Mathematics, 23.09.2021 23:40








Mathematics, 23.09.2021 23:40

Business, 23.09.2021 23:40

History, 23.09.2021 23:40

English, 23.09.2021 23:40



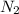 = 8.00 atm
= 8.00 atm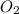 = 5.00 atm
= 5.00 atm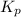 = 0.0025
= 0.0025