
Chemistry, 13.11.2019 06:31 PrincessKehlani15
The oxidation of glucose can result in several products. you perform combustion analysis on a 2.750 g sample that resulted from the oxidation of glucose and obtain 3.702 g co2 and 1.514 g h2o. how many carbon atoms are in the empirical formula of the isolated sample? (assume no other products present from the oxidation of glucose.)]

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 21.06.2019 21:30
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

You know the right answer?
The oxidation of glucose can result in several products. you perform combustion analysis on a 2.750...
Questions

French, 15.10.2020 21:01



World Languages, 15.10.2020 21:01


Mathematics, 15.10.2020 21:01

Mathematics, 15.10.2020 21:01




Mathematics, 15.10.2020 21:01



Biology, 15.10.2020 21:01

Mathematics, 15.10.2020 21:01


History, 15.10.2020 21:01

English, 15.10.2020 21:01


Mathematics, 15.10.2020 21:01

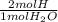 = 0.1682 mol H
= 0.1682 mol H

