
Consider the acid/base properties of iron(iii). a. find the ph of a solution made by dissolving 0.7438 g iron(iii) chloride hexahydrate in 200.00 ml of water using [fe(h2o)6] 3+ (aq) ⇌ [fe(h2o)5oh] 2+ (aq) + h+ (aq) pka = 2.83 b. the iron(iii) can be made to precipitate from the solution by addition of hydroxide to form iron(iii) hydroxide. at what ph will the iron(iii) concentration be reduced to 1.0×10−10 m?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 21:50
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l.s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2
You know the right answer?
Consider the acid/base properties of iron(iii). a. find the ph of a solution made by dissolving 0.74...
Questions

English, 10.09.2019 05:30

















Computers and Technology, 10.09.2019 05:30

Mathematics, 10.09.2019 05:30

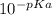
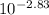
![Ka = \frac{[H^+]*[[Fe(H2O)5OH]^{+2}]}{[[Fe(H2O)6]^{+3}]}](/tpl/images/0369/9679/898a6.png)


