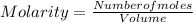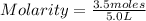What is the molarity of a solution containing 3.5 moles of hcl in 5.0 l of water?
a) 0....


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1
You know the right answer?
Questions


Biology, 17.02.2021 14:10


History, 17.02.2021 14:10



English, 17.02.2021 14:10


Arts, 17.02.2021 14:10



English, 17.02.2021 14:10

English, 17.02.2021 14:10

Mathematics, 17.02.2021 14:10

History, 17.02.2021 14:10

Mathematics, 17.02.2021 14:10



Mathematics, 17.02.2021 14:10