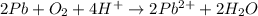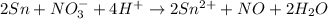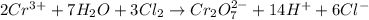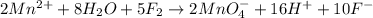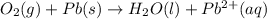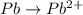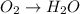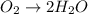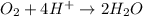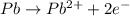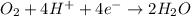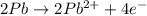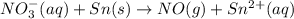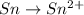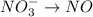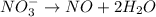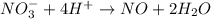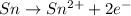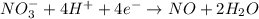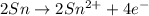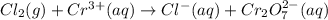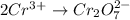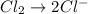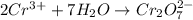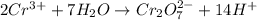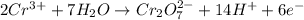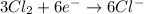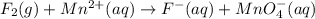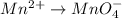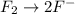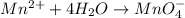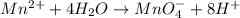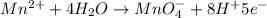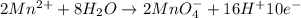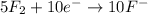Balance the following oxidation-reduction reactions, which occur in acidic solution, using the half-reaction method.
(use the lowest possible coefficients. include states-of-matter under the given conditions in your answer.)
o2(g) + pb(s) → h2o(l) + pb2+(aq) (b) no3−(aq) + sn(s) → no(g) + sn2+(aq) (c) cl2(g) + cr3+(aq) → cl −(aq) + cr2o72−(aq) (d) f2(g) + mn2+(aq) → f −(aq) + mno4−(aq)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

You know the right answer?
Balance the following oxidation-reduction reactions, which occur in acidic solution, using the half-...
Questions



Mathematics, 25.11.2021 07:20

Mathematics, 25.11.2021 07:20


Biology, 25.11.2021 07:20


History, 25.11.2021 07:20



Mathematics, 25.11.2021 07:20







Health, 25.11.2021 07:20

Social Studies, 25.11.2021 07:20