
Cobalt(ii) cation can combine with other small ions besides the chloride anion studied in lab 2. consider the following equilibrium reaction and the experimental data collected for it. co(h2o)6]2 (aq) 4 scn-(aq) ⇌ [co(scn)4]2-(aq) 6 h2o(l) experimental data: placed in a hot water bath, the solution turns blue, which is the color of the [co(scn)4]2-(aq) complex. placed in an ice bath, the solution turns light pink, which is the color of the co(h2o)6]2 (aq) complex. should the "heat" term be placed on the reactant side with the [co(h2o)6]2 or on the product side with the [co(scn)4]2-write a balanced chemical equation that describes the equilibrium reaction of the hexaaquocobalt(ii) and tetrachlorocobalt(ii) complex ions, in which the tetrachlorocobalt(ii) species is a product. to do this, think about the following questions: what are you adding to the hexaaquocobalt(ii) complex to convert it to the tetrachlorocobalt(ii) complex? what molecule is displaced when the tetrachlorocobalt(ii) complex is made?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Hot air balloons float in the air because of the difference in density between cold and hot air. in this problem, you will estimate the minimum temperature the gas inside the balloon needs to be, for it to take off. to do this, use the following variables and make these assumptions: the combined weight of the pilot basket together with that of the balloon fabric and other equipment is w. the volume of the hot air inside the balloon when it is inflated is v. the absolute temperature of the hot air at the bottom of the balloon is th (where th> tc). the absolute temperature of the cold air outside the balloon is tc and its density is ďc. the balloon is open at the bottom, so that the pressure inside and outside the balloon is the same. as always, treat air as an ideal gas. use g for the magnitude of the acceleration due to gravity.
Answers: 1

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

You know the right answer?
Cobalt(ii) cation can combine with other small ions besides the chloride anion studied in lab 2. con...
Questions




Chemistry, 13.12.2020 21:20

Arts, 13.12.2020 21:20

History, 13.12.2020 21:20

English, 13.12.2020 21:20


Mathematics, 13.12.2020 21:20

Health, 13.12.2020 21:20


Mathematics, 13.12.2020 21:20

Arts, 13.12.2020 21:20


Mathematics, 13.12.2020 21:20


Mathematics, 13.12.2020 21:20


Biology, 13.12.2020 21:20

History, 13.12.2020 21:20

![\rm [Co(H_2O)_6]^{2+}](/tpl/images/0366/6179/9193b.png) and
and 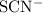 .
.![\rm [Co(H_2O)_6]^{2+}\; (aq) + 4\; SCN^{-1}\;(aq) \rightleftharpoons [Co(SCN)_4]^{2-}\; (aq) + 6\; H_2O\; (aq)](/tpl/images/0366/6179/683e4.png) .
.![[Co(SCN)_4]^{2-}](/tpl/images/0366/6179/5bfb1.png) is the one that absorbs heat. As a result, "heat" should be placed on that side of the equation that comes with
is the one that absorbs heat. As a result, "heat" should be placed on that side of the equation that comes with ![\rm [Co(SCN)_4]^{2-}](/tpl/images/0366/6179/9633f.png) ions.Water molecules displace
ions.Water molecules displace 

