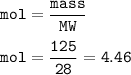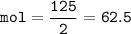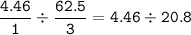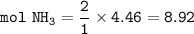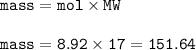Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1
You know the right answer?
If 125.0g of nitrogen is reacted with 125.0g of hydrogen, what is the theoretical yield of the react...
Questions

Mathematics, 17.01.2022 14:00

Mathematics, 17.01.2022 14:00

Mathematics, 17.01.2022 14:00

World Languages, 17.01.2022 14:00

Mathematics, 17.01.2022 14:00

Mathematics, 17.01.2022 14:00


Mathematics, 17.01.2022 14:00

Geography, 17.01.2022 14:00


Mathematics, 17.01.2022 14:00


Arts, 17.01.2022 14:00

World Languages, 17.01.2022 14:00

Business, 17.01.2022 14:00




Mathematics, 17.01.2022 14:00

Mathematics, 17.01.2022 14:00