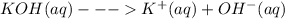Chemistry, 06.11.2019 06:31 AmyGonzalez1385
Consider the dissolution of mns in water (ksp = 3.0 × 10–14). mns(s) + h2o(l) mn2+(aq) + hs–(aq) + oh–(aq) how is the solubility of manganese(ii) sulfide affected by the addition of aqueous potassium hydroxide to the system?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1


You know the right answer?
Consider the dissolution of mns in water (ksp = 3.0 × 10–14). mns(s) + h2o(l) mn2+(aq) + hs–(aq) + o...
Questions

Mathematics, 15.01.2021 20:50





Mathematics, 15.01.2021 20:50




Mathematics, 15.01.2021 20:50



Mathematics, 15.01.2021 20:50





Mathematics, 15.01.2021 20:50