
Chemistry, 06.11.2019 06:31 maggie123433
The aluminum cup inside your calorimeter weighs 41.55 g. you add 59.21 g of 1.0 m acetic acid solution and 50.03 g of 1.0 m sodium hydroxide solution to the calorimeter. both solutions have an initial temperature of 19.9 oc, and the final temperature after addition is 26.8 oc. what is the molar enthalpy of neutralization, in units of kj/mol? assume that: the calorimeter is completely insulated the heat capacity of the empty calorimeter is the heat capacity of the aluminum cup: 0.903 j g-1 oc-1. the density of the two solutions is the same as that of water: 1.00 g/ml. the heat capacity of the two solutions is the same as that of water: 4.184 j g-1 oc-1.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1


You know the right answer?
The aluminum cup inside your calorimeter weighs 41.55 g. you add 59.21 g of 1.0 m acetic acid soluti...
Questions

Chemistry, 11.06.2021 22:50


Social Studies, 11.06.2021 22:50

Health, 11.06.2021 22:50

Mathematics, 11.06.2021 22:50



Biology, 11.06.2021 22:50

Mathematics, 11.06.2021 22:50

Biology, 11.06.2021 22:50



Mathematics, 11.06.2021 22:50



Mathematics, 11.06.2021 22:50



History, 11.06.2021 22:50

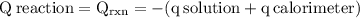
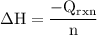
![\rm \DeltaH=-[\dfrac{- 3412.6007}{0.05003}]\\\\\Delta H=\:68211.087\dfrac{J}{mole} =68.211\dfrac{kJ}{mol}](/tpl/images/0361/7451/7b811.png)



