
Chemistry, 06.11.2019 02:31 kimbely7704
The molar heat capacity of ethane is represented in the temperature range 298 k to 400 k by the empirical expression cp, m in j k1 mol 14.73 + (0.1272 t in k). the corresponding expressions for c(e) and h2(g) are given in the back of the atkins textbook. calculate the standard enthalpy of formation of ethane at 373 k from its value at 298 k, in kj mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

You know the right answer?
The molar heat capacity of ethane is represented in the temperature range 298 k to 400 k by the empi...
Questions

Social Studies, 16.10.2019 10:30


Mathematics, 16.10.2019 10:30

History, 16.10.2019 10:30


Health, 16.10.2019 10:30


Mathematics, 16.10.2019 10:30


History, 16.10.2019 10:30

Biology, 16.10.2019 10:30




English, 16.10.2019 10:30

Mathematics, 16.10.2019 10:30

History, 16.10.2019 10:30

Mathematics, 16.10.2019 10:30

Health, 16.10.2019 10:30

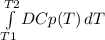
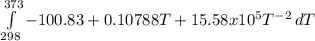 = -3796.48 J/mol = -3.80 kJ/mol (solved by a graphic calculator)
= -3796.48 J/mol = -3.80 kJ/mol (solved by a graphic calculator)

