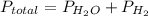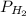Chemistry, 05.11.2019 02:31 jitosfc916
If the vapor pressure of water ( ) at 20.0°c is 17.5 mm hg, and the atmospheric pressure measured by a barometer (
) at 20.0°c is 17.5 mm hg, and the atmospheric pressure measured by a barometer ( ) was 757.3 mm hg, what is the partial pressure of h₂ gas (
) was 757.3 mm hg, what is the partial pressure of h₂ gas ( ) in the gas mixture in a buret? (use dalton’s law of partial pressures to solve for
) in the gas mixture in a buret? (use dalton’s law of partial pressures to solve for  )
)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

You know the right answer?
If the vapor pressure of water ([tex]p_{h_2o}[/tex]) at 20.0°c is 17.5 mm hg, and the atmospheric pr...
Questions

Physics, 30.08.2019 07:30



Mathematics, 30.08.2019 07:30


Mathematics, 30.08.2019 07:30



History, 30.08.2019 07:30



Mathematics, 30.08.2019 07:30


Biology, 30.08.2019 07:30

Mathematics, 30.08.2019 07:30