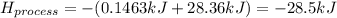Chemistry, 01.11.2019 02:31 tintlemax6256
When 50.0 ml of water containing 0.50 mol hcl at 22.5°c are mixed with 50.0 ml of water containing 0.50 mol naoh at 22.5°c in a calorimeter, the temperature of the solution increases to 26.0°c. how much heat (in kj) was released by this reaction? note: the specific heat of water (cwater) is 4.18 j/(g•˚c) and the density of the solution is 1.00 g/ml.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2
You know the right answer?
When 50.0 ml of water containing 0.50 mol hcl at 22.5°c are mixed with 50.0 ml of water containing 0...
Questions




Mathematics, 04.12.2019 21:31






Computers and Technology, 04.12.2019 21:31


Mathematics, 04.12.2019 21:31



History, 04.12.2019 21:31


Biology, 04.12.2019 21:31



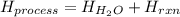
![H_{H_2O}=[(50.0mL+50mL)*\frac{1g}{1mL}]*4.18\frac{J}{mol^0C}*(26.0-22.5)^0C\\H_{H_2O}=146.3J=0.1463kJ](/tpl/images/0355/0596/d18d3.png)