
Chemistry, 26.10.2019 03:43 lordcaos066
Write the henderson-hasselbalch equation for a propanoic acid solution ( ch3ch2co2h , pka=4.874 ) using the symbols ha and a− , and the given pka value for propanoic acid in the expression. ph= 4.874 + log [a-]/[ha] using the equation to calculate the quotient [a−] / [ha] at three different ph values.(a) ph = 4.23(b) ph = 4.87(c) ph = 5.3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1


Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
Write the henderson-hasselbalch equation for a propanoic acid solution ( ch3ch2co2h , pka=4.874 ) us...
Questions

Mathematics, 06.01.2021 21:40






Biology, 06.01.2021 21:40


Mathematics, 06.01.2021 21:40

Mathematics, 06.01.2021 21:40


English, 06.01.2021 21:40

Chemistry, 06.01.2021 21:40

Mathematics, 06.01.2021 21:40

Mathematics, 06.01.2021 21:40

Mathematics, 06.01.2021 21:40


Mathematics, 06.01.2021 21:40


Spanish, 06.01.2021 21:40

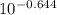 = [A⁻]/[HA]
= [A⁻]/[HA]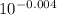 = [A⁻]/[HA]
= [A⁻]/[HA]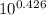 = [A⁻]/[HA]
= [A⁻]/[HA]

