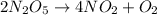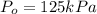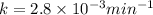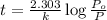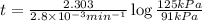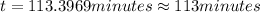The decomposition of dinitrogen pentoxide, n2o5, to no2 and o2 is a first-order reaction. at 60°c, the rate constant is 2.8 × 10-3min-1. if a rigid vessel initially contains only n2o5 at a pressure of 125 kpa, how long will it take for the total pressure to reach 176 kpa? options (pick 1)a)113 minb)129 minc)42 mind)182 mine)62 minf)83 min

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Which is true of the reactants in this displacement reaction? fe + 2hcl fecl2 + h2 a. the reactants are located to the left of the arrow in the chemical equation. b. the reactants contain 1 iron atom, 2 hydrogen atoms, and 1 chlorine atom. c. the reactants are the atoms, molecules, or compounds formed in the reaction. d. the reactants have the same physical and chemical properties as the products.
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2


Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2
You know the right answer?
The decomposition of dinitrogen pentoxide, n2o5, to no2 and o2 is a first-order reaction. at 60°c, t...
Questions

Mathematics, 18.01.2021 22:10

Arts, 18.01.2021 22:10





Mathematics, 18.01.2021 22:10




Mathematics, 18.01.2021 22:10

English, 18.01.2021 22:10





Geography, 18.01.2021 22:10