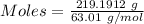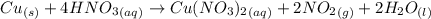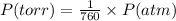Nitrogen dioxide is used industrially to produce nitric acid, but it contributes to acid rain and photochemical smog. what volume of nitrogen dioxide is formed at 724 torr and 28.2° c by reacting 4.84 cm3 of copper (d = 8.95 g/cm3) with 227 ml of nitric acid (d = 1.42 g/cm3, 68.0% hno3 by mass)? cu + 4hno3 = cu(no3)2 + 2no2 + 2h2o

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1


Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
Nitrogen dioxide is used industrially to produce nitric acid, but it contributes to acid rain and ph...
Questions

Computers and Technology, 23.11.2020 23:20



Social Studies, 23.11.2020 23:20

Health, 23.11.2020 23:20



History, 23.11.2020 23:30


Biology, 23.11.2020 23:30

Mathematics, 23.11.2020 23:30

Mathematics, 23.11.2020 23:30

Mathematics, 23.11.2020 23:30

Biology, 23.11.2020 23:30





Social Studies, 23.11.2020 23:30

Mathematics, 23.11.2020 23:30

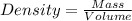
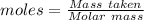
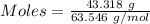
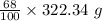 = 219.1912 g
= 219.1912 g