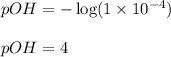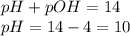Chemistry, 23.10.2019 21:00 janelisse199820
Asolution contains 1 latex: \times\: ×10−4 m oh– ions. calculate the solution ph value, and determine if the solution is acidic, basic, or neutral. math formulas: latex: ph=-\log\left[h_3o^+\right]p h = − log [ h 3 o + ] latex: poh=-\log\left[oh^-\right]p o h = − log [ o h − ] latex: ph+poh=14.00p h + p o h = 14.00 latex: \left[h_3o^+\right]\left[oh^-\right ]=1.0\times10^{-14}

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

You know the right answer?
Asolution contains 1 latex: \times\: ×10−4 m oh– ions. calculate the solution ph value, and determi...
Questions


Mathematics, 20.09.2020 09:01

English, 20.09.2020 09:01



Mathematics, 20.09.2020 09:01



Chemistry, 20.09.2020 09:01


English, 20.09.2020 09:01




Law, 20.09.2020 09:01



Geography, 20.09.2020 09:01


![pOH=-\log[OH^-]](/tpl/images/0343/2369/fe336.png)
![[OH^-]=1\times 10^{-4}M](/tpl/images/0343/2369/525b6.png)