Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

You know the right answer?
Phosphine, an extremely poisonous and highly reactive gas, will react with oxygen to form tetraphosp...
Questions

Computers and Technology, 28.09.2019 20:30


Mathematics, 28.09.2019 20:30

History, 28.09.2019 20:30

Spanish, 28.09.2019 20:30

Biology, 28.09.2019 20:30


Mathematics, 28.09.2019 20:30


Mathematics, 28.09.2019 20:30


Geography, 28.09.2019 20:30


Social Studies, 28.09.2019 20:30

History, 28.09.2019 20:30

History, 28.09.2019 20:30

English, 28.09.2019 20:30



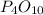 formed is 469.8 grams.
formed is 469.8 grams.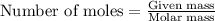 ......(1)
......(1)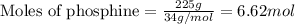
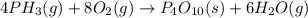
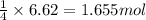 of
of