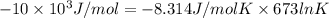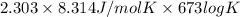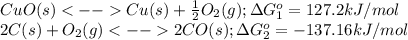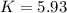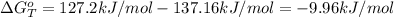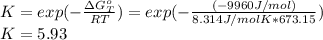Chemistry, 19.10.2019 02:30 patrickgonzalezjr13
Heating copper(ii) oxide at 400°c does not produce any appreciable amount of cu: cuo(s) ⇆ cu(s) + 1 2 o2(g) δg o = 127.2 kj/mol however, if this reaction is coupled to the conversion of graphite to carbon monoxide, it becomes spontaneous. write an equation for the coupled process and calculate the equilibrium constant for the coupled reaction. be sure to include the states of the chemical species.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1


Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
You know the right answer?
Heating copper(ii) oxide at 400°c does not produce any appreciable amount of cu: cuo(s) ⇆ cu(s) + 1...
Questions



English, 27.09.2019 12:00

Computers and Technology, 27.09.2019 12:00


Mathematics, 27.09.2019 12:00

Social Studies, 27.09.2019 12:00



Chemistry, 27.09.2019 12:00


Arts, 27.09.2019 12:00




Social Studies, 27.09.2019 12:00

History, 27.09.2019 12:00


Mathematics, 27.09.2019 12:00

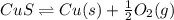
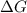 is positive. Therefore, reaction is non-spontaneous.
is positive. Therefore, reaction is non-spontaneous.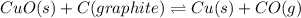
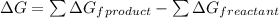
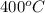 = (400 + 273) K = 673 K
= (400 + 273) K = 673 K