
Chemistry, 19.10.2019 00:30 laequity7325
Sodium thiosulfate, na2s2o3, is used as a fixer in photographic film developing. the amount of na2s2o3 in a solution can be determined by a titration with iodine, i2, according to the equation: 2na2s2o3(aq) + i2(aq) --> na2s4o6 +2nai(aq). calculate the concentration of the na2s2o3 solution if 23.50 ml of a 0.1710 m i2 solution react exactly with a 100.0 ml sample of the na2s2o3 solution. use 4 sig. fig. aside, the end of the titration is determined by the color. nai is a pale yellow and i2 is a deep purple. just when the purple color persists, all the iodine that can react has reacted.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 23.06.2019 07:30
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes no correct anwser get brainliest
Answers: 1
You know the right answer?
Sodium thiosulfate, na2s2o3, is used as a fixer in photographic film developing. the amount of na2s2...
Questions

Business, 01.12.2021 09:20





Physics, 01.12.2021 09:20


History, 01.12.2021 09:20



Biology, 01.12.2021 09:20



Mathematics, 01.12.2021 09:20

Mathematics, 01.12.2021 09:20




Mathematics, 01.12.2021 09:20

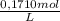 = 4,0185x10⁻³ moles of I₂
= 4,0185x10⁻³ moles of I₂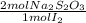 = 8,037x10⁻³ moles of Na₂S₂O₃
= 8,037x10⁻³ moles of Na₂S₂O₃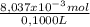 = 0,08037 M
= 0,08037 M

