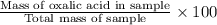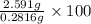Chemistry, 19.10.2019 00:30 reagriffis24
Oxalic acid, a diprotic acid having the formula h2c2o4, is used to clean the rust out of radiators in cars. a sample of an oxalic acid mixture was analyzed by titrating a 0.2816 g sample dissolved in water with 0.0461 m naoh. a volume of 11.49 ml of the base was required to completely neutralize the oxalic acid. what was the percentage by mass of oxalic acid in the sample?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:00
__ _ _ _ _ is the process of removing earth materials from their original sites through weathering and transport and depositing the in another location. a. erosion b. sedimentation c. lithification d. dissolution
Answers: 1


Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2
You know the right answer?
Oxalic acid, a diprotic acid having the formula h2c2o4, is used to clean the rust out of radiators i...
Questions




Mathematics, 17.06.2021 04:00

Physics, 17.06.2021 04:00

Biology, 17.06.2021 04:00







Mathematics, 17.06.2021 04:00


History, 17.06.2021 04:00





Advanced Placement (AP), 17.06.2021 04:00

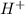 ions produced by
ions produced by 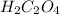 with total number of moles of
with total number of moles of 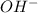 ions produced by NaOH.
ions produced by NaOH.
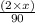 ........... (1) (We multiply by 2 because Oxalic Acid is a diprotic acid)
........... (1) (We multiply by 2 because Oxalic Acid is a diprotic acid)