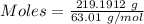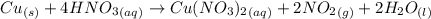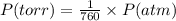Chemistry, 18.10.2019 19:10 densliverdensentos
Nitrogen dioxide is used industrially to produce nitric acid, but it contributes to acid rain and photochemical smog. what volume of nitrogen dioxide is formed at 724 torr and 28.2° c by reacting 4.84 cm3 of copper (d = 8.95 g/cm3) with 227 ml of nitric acid (d = 1.42 g/cm3, 68.0% hno3 by mass)?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 23.06.2019 02:50
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2

Chemistry, 23.06.2019 08:00
Amechanical wave that transports a lot of energy will have a
Answers: 2
You know the right answer?
Nitrogen dioxide is used industrially to produce nitric acid, but it contributes to acid rain and ph...
Questions

Mathematics, 26.04.2021 14:10






Mathematics, 26.04.2021 14:10


Mathematics, 26.04.2021 14:10


Social Studies, 26.04.2021 14:10

English, 26.04.2021 14:10



Mathematics, 26.04.2021 14:10

Social Studies, 26.04.2021 14:10




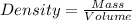
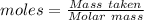
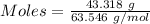
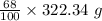 = 219.1912 g
= 219.1912 g