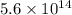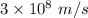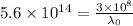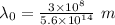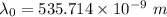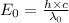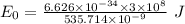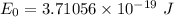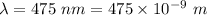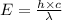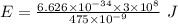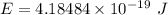Chemistry, 18.10.2019 03:30 garcikyl000
Would a photon with a wavelength of 475nm have enough energy to eject an electron from metal x? (frequency threshold= 5.6x10^14hz) (wavelength= 3.7x10^-19j)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
Would a photon with a wavelength of 475nm have enough energy to eject an electron from metal x? (fr...
Questions

Mathematics, 03.02.2020 17:43

Mathematics, 03.02.2020 17:43

Biology, 03.02.2020 17:43



Mathematics, 03.02.2020 17:43



History, 03.02.2020 17:44

Social Studies, 03.02.2020 17:44



English, 03.02.2020 17:44

English, 03.02.2020 17:44

Physics, 03.02.2020 17:44





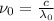
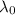 is the threshold wavelength
is the threshold wavelength
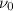 is the threshold frequency =
is the threshold frequency =