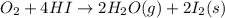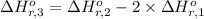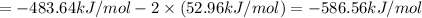Chemistry, 18.10.2019 03:30 miya257916
Calculate the standard enthalpy of the 3rd reaction using the given data: h2(g) +126) +2 hio) a, hº = +52.96 kj/mol 2 h2(g) + o2(g) + 2 h209) a, hº = - 483.64 kj/mol 4 hi) + o2(g) +2 12(8) + 2 h206) a, hº =? a. h=-589.5619/mol calculate the internal energy change for reaction (3) in the previous problem.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

You know the right answer?
Calculate the standard enthalpy of the 3rd reaction using the given data: h2(g) +126) +2 hio) a, hº...
Questions


Mathematics, 21.04.2021 20:00

History, 21.04.2021 20:00

Biology, 21.04.2021 20:00

Advanced Placement (AP), 21.04.2021 20:00



Mathematics, 21.04.2021 20:00

Mathematics, 21.04.2021 20:00




Mathematics, 21.04.2021 20:00

Computers and Technology, 21.04.2021 20:00

Computers and Technology, 21.04.2021 20:00

Mathematics, 21.04.2021 20:00

Mathematics, 21.04.2021 20:00


History, 21.04.2021 20:00

 ...[1]
...[1]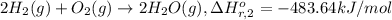 ...[2]
...[2]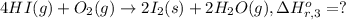 ..[3]
..[3]