Chemistry, 18.10.2019 03:30 GamerGirl15
4. copper metal reacts with nitric acid(hno3) to produce aqueous copper (ii)nitrate, nitrogen dioxide gas and liquid water. a. write the balanced equation for the reaction b. if there are 0.500 moles of copper metal present how many moles of nitric acid are required for the reaction? how many moles of nitrogen dioxide gas are formed?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2


Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1
You know the right answer?
4. copper metal reacts with nitric acid(hno3) to produce aqueous copper (ii)nitrate, nitrogen dioxid...
Questions

Social Studies, 27.09.2019 07:30

Biology, 27.09.2019 07:30

Physics, 27.09.2019 07:30


Biology, 27.09.2019 07:30




Mathematics, 27.09.2019 07:30

History, 27.09.2019 07:30


Mathematics, 27.09.2019 07:30


Mathematics, 27.09.2019 07:30




Chemistry, 27.09.2019 07:30


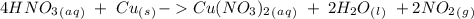
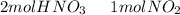
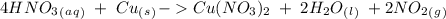
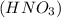 we have to use the molar ratio in the balence reaction:
we have to use the molar ratio in the balence reaction: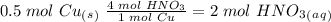
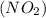 we have to follow the same logic:
we have to follow the same logic: