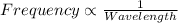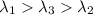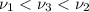One type of electromagnetic radiation has a frequency of 107.1 mhz, another type has a wavelength of 2.12 10-10 m, and another type of electromagnetic radiation has photons with energy equal to 3.97 10-19 j/photon. identify each type of electromagnetic radiation. 107.1 mhz 2.12 10-10 m 3.97 10-19 j/photon fm radiowaves x-rays visible (green) light visible (red) light fm radiowaves x-rays visible (green) light visible (red) light fm radiowaves x-rays visible (green) light visible (red) light rank them in order of increasing photon energy and increasing frequency

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3
You know the right answer?
One type of electromagnetic radiation has a frequency of 107.1 mhz, another type has a wavelength of...
Questions


Mathematics, 25.10.2021 17:30



English, 25.10.2021 17:30

Health, 25.10.2021 17:30

Social Studies, 25.10.2021 17:30

Mathematics, 25.10.2021 17:30

Mathematics, 25.10.2021 17:30


Computers and Technology, 25.10.2021 17:30




Mathematics, 25.10.2021 17:30

English, 25.10.2021 17:30

Mathematics, 25.10.2021 17:30

Mathematics, 25.10.2021 17:30

English, 25.10.2021 17:30

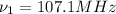
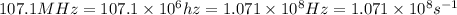
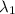
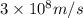
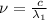
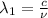
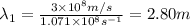
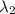
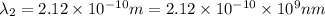
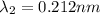 (X-rays)
(X-rays)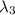
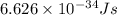
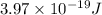
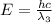
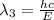
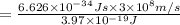
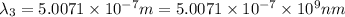
 (visible light)
(visible light)