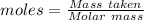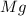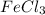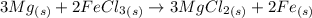Chemistry, 16.10.2019 06:00 jessica28757
Magnesium reacts with iron(iii) chloride to form magnesium chloride (which can be used in fireproofing wood and in disinfectants) and iron. 3mg(s) + 2fecl3(s) → 3mgcl2(s) + 2fe(s) a mixture of 41.0 g of magnesium ( = 24.31 g/mol) and 175 g of iron(iii) chloride ( = 162.2 g/mol) is allowed to react. what mass of magnesium chloride = 95.21 g/mol) is formed?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1
You know the right answer?
Magnesium reacts with iron(iii) chloride to form magnesium chloride (which can be used in fireproofi...
Questions


Social Studies, 24.09.2019 04:30


Mathematics, 24.09.2019 04:30


Mathematics, 24.09.2019 04:30








Mathematics, 24.09.2019 04:30




Computers and Technology, 24.09.2019 04:30