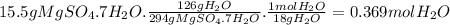Chemistry, 16.10.2019 01:30 Serenitybella
Bath salts such as epsom contain hydrate salts such as mgso 4 ·7h 2 o (magnesium sulfate heptahydrate). when heated, water is released as vapor and anhydrous mgso 4 remains. determine how many moles of vapor are released when 15.5 g of mgso 4 ·7h 2 o are heated. hint: include h 2 o when calculating the molar mass.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 23.06.2019 02:30
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2
You know the right answer?
Bath salts such as epsom contain hydrate salts such as mgso 4 ·7h 2 o (magnesium sulfate heptahydrat...
Questions




Biology, 10.12.2020 18:40

History, 10.12.2020 18:40

Health, 10.12.2020 18:40


Mathematics, 10.12.2020 18:40

Mathematics, 10.12.2020 18:40

Mathematics, 10.12.2020 18:40



Mathematics, 10.12.2020 18:40

Spanish, 10.12.2020 18:40

Biology, 10.12.2020 18:40

Mathematics, 10.12.2020 18:40


History, 10.12.2020 18:40

Mathematics, 10.12.2020 18:40

Biology, 10.12.2020 18:40