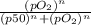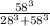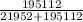Chemistry, 15.10.2019 05:30 skybrinson04
Calculate the fractional saturation for hemoglobin when the partial pressure of oxygen is 58 mm hg. assume hemoglobin is 50% saturated with oxygen at a partial pressure of 28 mm hg and that the hill coefficient is 3. express your answer to two significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3
You know the right answer?
Calculate the fractional saturation for hemoglobin when the partial pressure of oxygen is 58 mm hg....
Questions


Mathematics, 09.12.2021 01:00



Mathematics, 09.12.2021 01:00




Chemistry, 09.12.2021 01:00

Business, 09.12.2021 01:00


History, 09.12.2021 01:00

French, 09.12.2021 01:00

Mathematics, 09.12.2021 01:00





Arts, 09.12.2021 01:00