Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1


Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
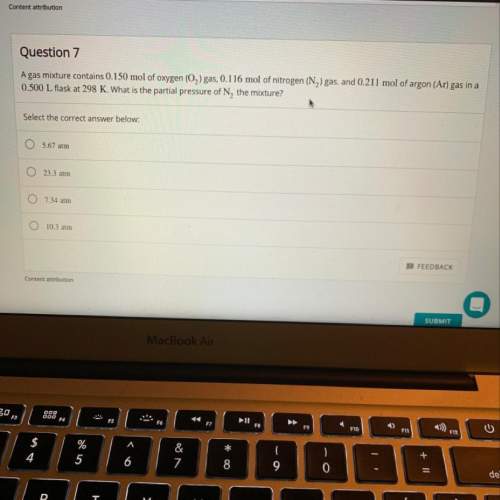
Agas mixture contains 0.150 mol of o2 gas, 0.116 mol of n2 gas, and 0.211 mol of ar gas in a 0.500 l...
Questions

Mathematics, 21.10.2020 04:01



Mathematics, 21.10.2020 04:01


History, 21.10.2020 04:01

English, 21.10.2020 04:01



Mathematics, 21.10.2020 04:01


Mathematics, 21.10.2020 04:01

History, 21.10.2020 04:01

Mathematics, 21.10.2020 04:01