Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1
You know the right answer?
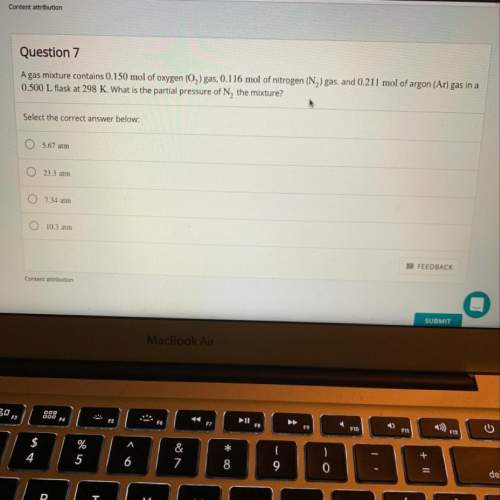
Agas mixture contains 0.150 mol of o2 gas, 0.116 mol of n2 gas, and 0.211 mol of ar gas in a 0.500 l...
Questions



History, 16.10.2019 22:10

German, 16.10.2019 22:10


Mathematics, 16.10.2019 22:10

History, 16.10.2019 22:10

Health, 16.10.2019 22:10




History, 16.10.2019 22:10





History, 16.10.2019 22:10


English, 16.10.2019 22:10

German, 16.10.2019 22:10